Trial overview
Staquis® was studied as a monotherapy in patients with mild-to-moderate AD, across two randomised, multicentre,
double-blind and vehicle-controlled trials lasting 28 days.
3
A total of 1,527 patients ≥2 years old were randomised 2:1 to receive Staquis® BID or vehicle (Studies: AD-301 [N=763; NCT02118766]; AD-302 [N=764; NCT02118792]).3
Study design
Key inclusion criteria3
- Age 2 years and older, with a clinical diagnosis of AD
- At least 5% treatable body surface area (BSA) involvement
- Baseline Investigator’s Static Global Assessment (ISGA) scale of Mild (2) or Moderate (3)
- Any previous use of biologic therapy
- Systemic corticosteroid or immunosuppressant use within 28 days prior to the study
- Topical corticosteroid (TCS) or topical calcineurin inhibitor (TCI) use within 14 days prior to the study
- Any active skin infections
Both trials involved topical application of a thin layer of Staquis® or vehicle to the affected area(s) twice daily, for 28 days.3
Primary/secondary endpoints
The primary endpoint of the Staquis® trials was success in ISGA score at Day 29, defined as a score of Clear
(0) or Almost Clear (1) AND a 2-grade or greater improvement from
baseline.3
ISGA score is a 5-point scale used to assess a patient’s overall disease severity across all treatable lesions.3
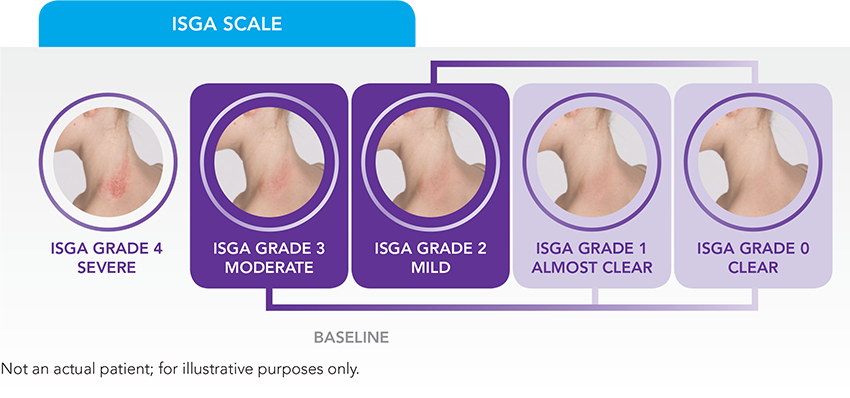
Secondary endpoints3
- Time to success in ISGA scale
- Proportion of patients achieving ISGA score of Clear (0) or Almost Clear (1)
Other pre-specified endpoints
- Assessment of pruritus severity and signs of AD (erythema, exudation, excoriation, induration/papulation, and lichenification).3
Exploratory endpoints (post-hoc analysis)
- Time to improvement in pruritus, defined as achieving a SPS score of ≤1 with at least 1-point improvement from baseline.7
- Proportion of subject with improvement in pruritus, defined as achieving a daily mean SPS score of ≤1 with at least 1-point improvement from baseline.7
- Change from baseline in mean dermatology-related
QOL scores6
- Children’s Dermatology Life Quality Index (CDLQI)
- Dermatology Life Quality Index (DLQI)
ISGA scale patient baseline profiles
Baseline characteristics of Staquis® and vehicle patients across both trials9
- 86% were <18 years of age
- 39% of subjects had baseline ISGA score of Mild (2)
- 61% of subjects had baseline ISGA score of Moderate (3)
- Mean treatable body surface area involvement ~18%
- 39% of subjects were non-Caucasian
Both treatment-naïve† and treatment-experienced patients with the
appropriate washout period were
included.9
ISGA = Investigator’s static global assessment; TCS = topical corticosteroid; TCI = topical calcineurin
inhibitor.
†Defined as any prior use of systemic or topical corticosteroids or TCI for the treatment of
AD
within 90 days before starting the study.9
‡In the pivotal trials races other than Caucasian included American Indian or Alaska Native,
Asian, Black or African American, Native Hawaii or other Pacific Islander and other.9


