Overview
Introduction Staquis® is the first non-steroidal, topical, phosphodiesterase 4 (PDE4) inhibitor indicated for topical treatment of mild to moderate atopic dermatitis in adult and pediatric patients 3 months of age and older.1
AD is a common chronic inflammatory skin condition characterized by skin barrier disruption and immune system abnormalities.10
Mechanism of disease
Mechanism of disease AD is associated with excess
production of proinflammatory
cytokines.10
The production of some cytokines is regulated by PDE4, which is often overactive in
cells (such as the leukocytes) of patients with AD.4
Overactive PDE4 increases the degradation of cyclic adenosine monophosphate (cAMP) to AMP, which leads to the overproduction of proinflammatory cytokines.3,5
Unmet need
Over 80% of children with AD have symptoms which continue into adulthood.2
Despite
many patients living with the condition for years,2 adherence to topical AD treatments is often poor
and might decreases over time.6
Topical treatments are commonly prescribed to alleviate symptoms, reduce inflammation and minimise
exacerbation.2
The American treatment recommendation from 2023 (AAD) treatment guidelines currently recommend the use of topical corticosteroids (TCS), topical calcineurin inhibitors (TCI), or PDE4 inhibitors in mild-moderate disease.2,8
Despite their efficacy, both TCS and TCI are associated with limitations such as application site reactions and
safety concerns with long-term application.
Some factors that should be considered when prescribing TCS
include
avoiding local cutaneous atrophy, treatable body surface area and sensitive skin regions.2
Understanding and evaluating AD
AD is chronic and a pruritic inflammatory skin condition that can affect both children and adults, with up to 90% of all patients presenting with a mild-to-moderate form of the disease.2
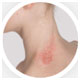
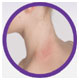
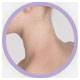
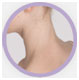
The Investigator’s Static Global Assessment (ISGA)‡ scale is a 5-point scale that can be used to assess the overall disease severity across all treatable lesions.2
| ISGA scale | Illustration† | ISGA scale definitions |
|---|---|---|
| ISGA Grade 4:
Severe |
|
|
| ISGA Grade 3:
Moderate |
|
|
| ISGA Grade 2:
Mild |
|
|
| ISGA Grade 1:
Almost Clear |
|
|
| ISGA Grade 0:
Clear |
|
|
†Not an actual patient; images for illustrative purposes only.
‡ISGA Grade 4 patients were excluded from Staquis® clinical trials.2
AD symptoms, such as pruritus, can also contribute to significant psychological, social and quality of life (QoL) burdens for both patients and caregivers.2,9
Footnotes: The specific mechanism(s) by which Staquis® exerts its therapeutic action is not well defined.1
Not an actual patient; for illustrative purposes only.
References
- Staquis® (crisaborole) latest approved prescribing information.
- Paller A, Tom W et al. J Am Acad Dermatol 2016;75(3):494–503.e6.
- Hanifin J, Chan S et al. J Invest Dermatol 1996;107(1):51–56.
- Chan S, Reifsnyder D et al. J Allergy Clin Immunol 1993;91(6):1179–1188.
- Jarnagin K, Chanda S et al. J Drugs Dermatol 2016;15(4):390–396.
- Tan X, Feldman S et al. Expert Opin Drug Deliv 2012;9(10):1263–1271.
- Fenerty S, O’Neill J et al. JAMA Dermatol 2013;149(2):229–231.
- Boguniewicz M, Fonacier L et al. Ann Allergy Asthma Immunol 2018;120(1):10–22.
- Yosipovich G, Simpson E et al. Itch 2018;3(2):e12.
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358(14):1483-1494