Most Common Adverse Reactions1-5
The most common adverse reactions (≥1%) of patients taking CIBINQO in placebo-controlled trials for up to 16 weeks1
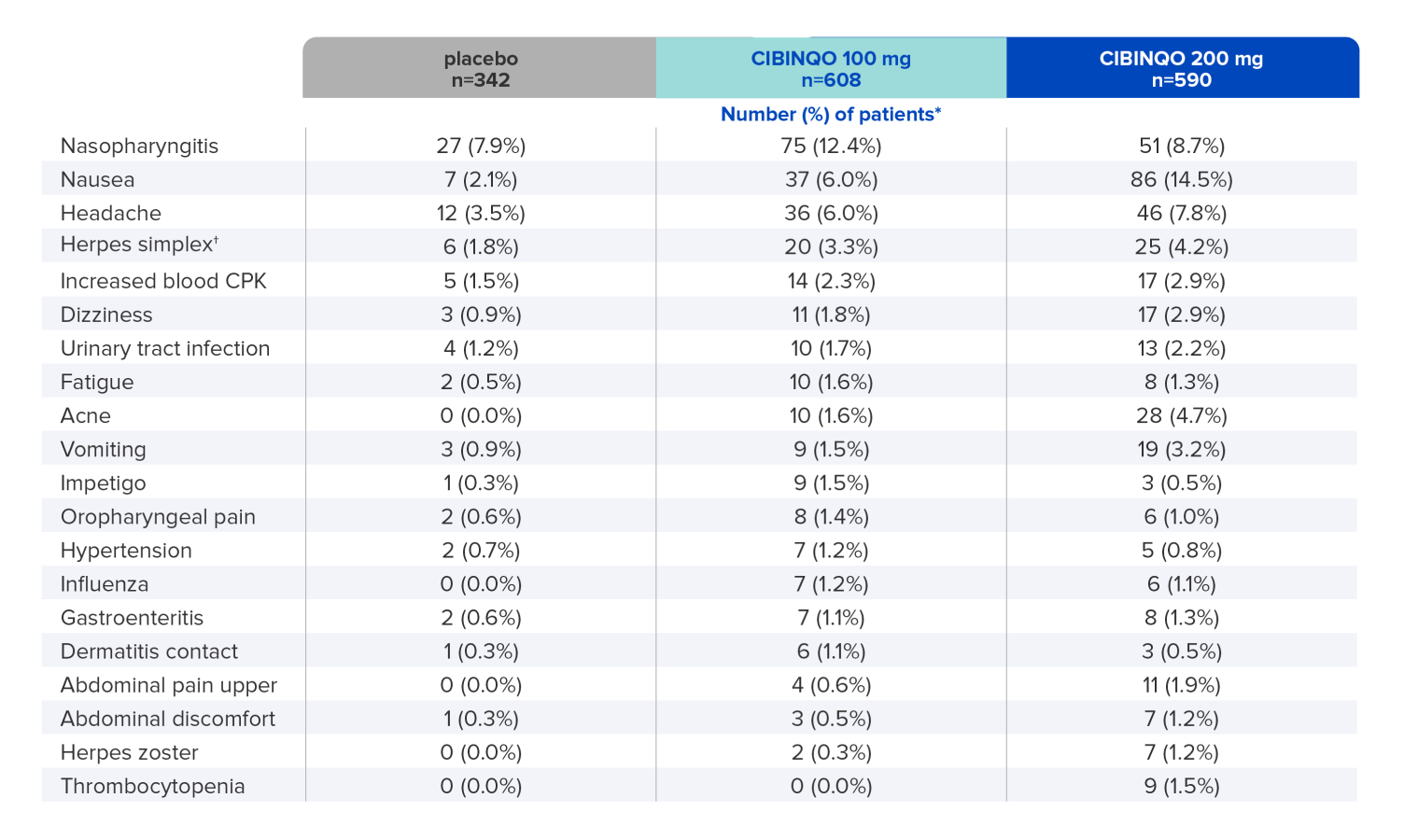
Data shown include one phase 2b and three phase 3 trials2.
The safety profile of CIBINQO in the monotherapy and combination trials was similar3-5.
* Study size adjusted percentages.
† Herpes simplex includes oral herpes, ophthalmic herpes, herpes dermatitis, and genital herpes.
CPK=creatinine phosphokinase.
Discontinuation rates due to adverse events for MONO-1, MONO-2, and COMPARE, respectively2

- The most frequent AE leading to discontinuation* was atopic dermatitis, occurring more frequently in the placebo treatment group (5.5%) than in patients taking either dose of CIBINQO (1.2%). Other frequent AEs leading to discontinuation, occurring more frequently in the CIBINQO group, were nausea (0.3%) and headache (0.2%)†
* Study size adjusted percentages.
† Data shown include one 2b and three phase 3 trials.
AE=adverse event.
References:
- Cibinqo (abrocitinib) latest approved Isareli prescribing information.
- Data on file. Pfizer Inc; New York, NY.
- Simpson EL, Silverberg JI, Nosbaum A, et al. Integrated safety analysis of abrocitinib for the treatment of moderate-to-severe atopic dermatitis from the phase II and phase III clinical trial program. Am J Clin Dermatol. 2021;22(5):693-707.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863-873.
- Bieber T, Simpson EL, Silverberg JI, et al; for the JADE COMPARE Investigators. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384(12):1101-1112.

