SERIOUS INFECTIONS
Serious infections have been reported in patients receiving abrocitinib. The most frequent serious infections in clinical studies were herpes simplex, herpes zoster and pneumonia.
Treatment must not be initiated in patients with an active, serious systemic infection.
Risks and benefits of treatment prior to initiating abrocitinib should be considered for patients:
- with chronic or recurrent infection
- who have been exposed to TB
- with a history of a serious or an opportunistic infection
- who have resided or travelled in areas of endemic TB or endemic mycoses; or
- with underlying conditions that may predispose them to infection.
MALIGNANCY
Malignancies, including non-melanoma skin cancer (NMSC), were observed in clinical studies with abrocitinib. Clinical data are insufficient to assess the potential relationship of exposure to abrocitinib and the development of malignancies. Long-term safety evaluations are ongoing.
The risks and benefits of abrocitinib treatment should be considered prior to initiating in patients with a known malignancy other than a successfully treated NMSC or cervical cancer in situ or when considering continuing therapy in patients who develop a malignancy. Periodic skin examination is recommended for patients who are at increased risk for skin cancer.
LIPIDS
Dose-dependent increases in blood lipid parameters were reported in patients treated with abrocitinib compared to placebo. Lipid parameters should be assessed approximately 4 weeks following initiation of therapy and thereafter according to the patient’s risk for cardiovascular disease. The effect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined. Patients with abnormal lipid parameters should be further monitored and managed according to clinical guidelines, due to the known cardiovascular risks associated with hyperlipidaemia. In patients with a high burden of cardiovascular risk factors, the risks and benefits of abrocitinib compared to that of other available therapies for atopic dermatitis should be considered. If abrocitinib is chosen, interventions to manage lipid concentrations should be implemented according to clinical guidelines.
Thrombotic events including pulmonary embolism
Events of deep venous thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients receiving abrocitinib. Abrocitinib should be used with caution in patients at high risk for DVT/PE. Risk factors that should be considered in determining the patient's risk for DVT/PE include older age, obesity, a medical history of DVT/PE, prothrombotic disorder, use of combined hormonal contraceptives or hormone replacement therapy, patients undergoing major surgery or prolonged immobilisation. If clinical features of DVT/PE occur, treatment should be discontinued and patients should be evaluated promptly, followed by appropriate treatment.
Other warnings include potential laboratory abnormalities. Avoid use of live vaccines during or immediately prior to treatment.
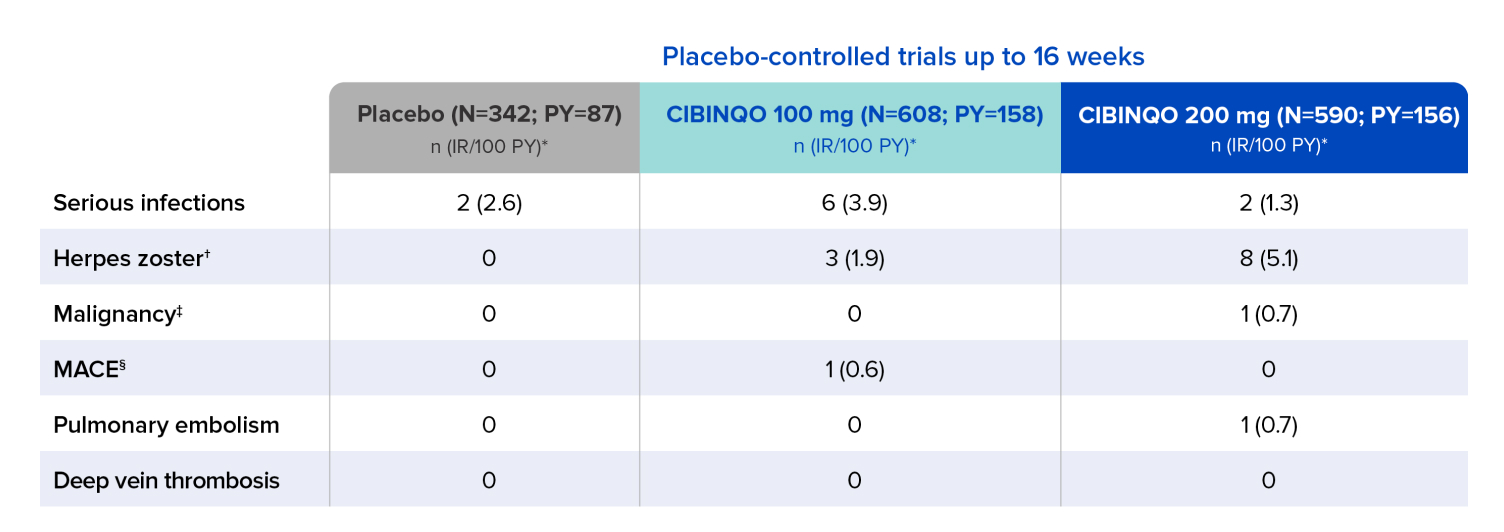
Malignancy
- Excluding NMSC: No cases were reported in subjects treated with placebo or either dose of CIBINQO
- NMSC: 1 subject treated with CIBINQO 200 mg
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The size of the control groups and duration of treatment do not permit precise comparative assessments for AEs with low frequency or long latency. In the chart where certain AEs have a zero, indicating no cases were observed in the reporting period, this should not be interpreted as no risk for a specified event.
Data shown includes one phase 2b and three phase 3 trials.
* Exposure adjusted incidence rates (IRs) were adjusted by trial for all the adverse reactions reported in this section. Patient-year is defined as the aggregate total follow-up time calculated for each event, censored at the time of first event for any given patient, subject to a risk period of up to 28 days beyond the last dose or to the data cutoff date.
† Opportunistic infections were generally cases of multidermatomal cutaneous herpes zoster.
‡ Includes all malignancy, including non-melanoma skin cancer (NMSC). Patients with history of malignancy except for treated NMSC and cervical carcinoma in situ were excluded from enrolling in trials.
§ MACE (major adverse cardiovascular event) defined as cardiovascular death, myocardial infarction, and stroke.
IR=incidence rate; PY=patient-years.
Well-studied safety profile in 3582 patients across multiple clinical trials, with some having exposure for ~2 years1-3
A long-term extension (LTE) safety study evaluating adults and adolescents aged 12 and older with moderate-to-severe AD who are taking CIBINQO, with or without Rx topical therapies.2
AEs of special interest
These data are from an integrated safety analysis consistent-dose cohort (n=2784) of JADE EXTEND; there was also a variable dose cohort that included additional safety data (n=798). 1451 patients treated with CIBINQO had at least 48 weeks of exposure, and 554 patients had at least 96 weeks of exposure. Data cutoff was April 16, 2021.*
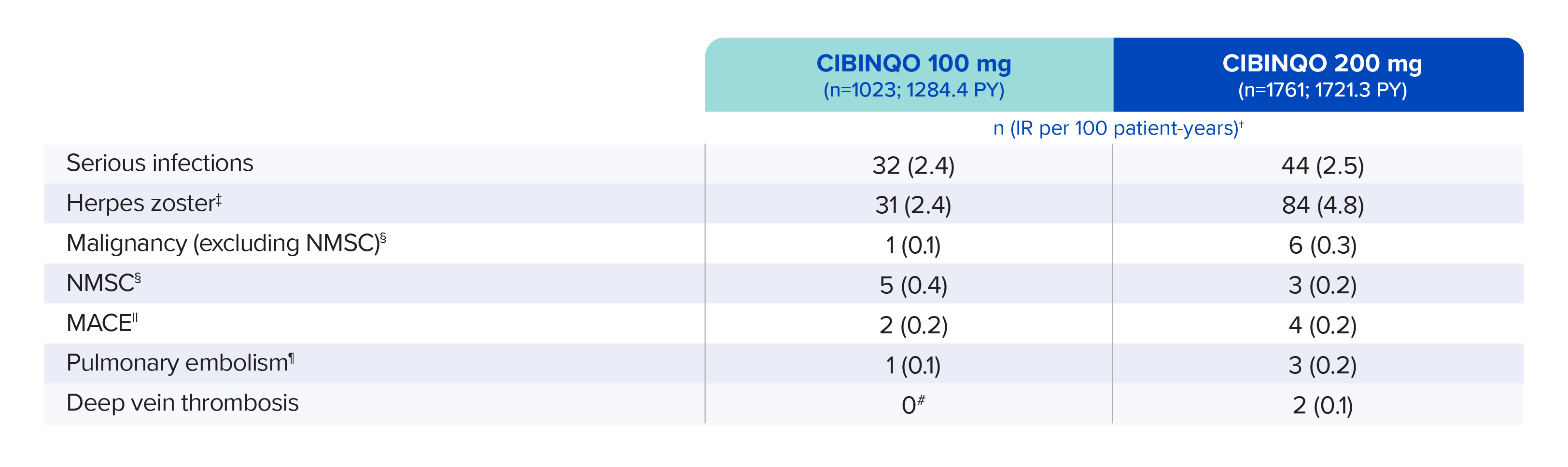
Patients receiving placebo were eligible to enroll in JADE EXTEND following completion of the qualifying JADE parent study; therefore, no patient received placebo after week 16.
Data shown includes one phase 2b and three phase 3 trials.
* Pooled safety from one phase 2b trial, six phase 3 trials, and one long-term extension (LTE) trial, which is ongoing. In the consistent-dose cohort, patients received the same CIBINQO dose during the entire exposure time. In the variable-dose cohort, patients received different doses of CIBINQO (100 mg or 200 mg) throughout the exposure time.
† Exposure-adjusted IRs were adjusted by trial for all of the adverse reactions reported in this section. Patient-year is defined as the aggregate total follow-up time calculated for each event, censored at the time of first event for any given patient, subject to a risk period of up to 28 days beyond the last dose or to the data cutoff date.
‡ Opportunistic infections were generally cases of nonserious multidermatomal herpes zoster. There was 1 event of adjudicated tuberculosis.
§ Patients with a history of malignancy, except for treated NMSC and cervical carcinoma in situ, were excluded from enrolling in the trials.
‖ MACE was defined as cardiovascular death, myocardial infarction, and stroke.
¶ One pulmonary embolism event (not adjudicated) was included.
# Confidence intervals (Cis) reflect the uncertainty around an estimated number; therefore, the upper limit (UL) is nonzero, reflecting that there is a chance that the true underlying IR could be slightly greater than zero with only a 2.5% chance (due to 95% CI) to be >0.47 for the incidence of PE and DVT in the CIBINQO 100 mg group, or >0.57 for the incidence of retinal detachment in the CIBINQO 200 mg group.
AE=adverse event; DVT=deep vein thrombosis; IR=incidence rate; MACE=major adverse cardiovascular event; NMSC=non-melanoma skin cancer; PY=patient-years.
JADE EXTEND study design1,2
A long-term extension (LTE) safety study evaluating adults and adolescents aged 12 and older with moderate-to-severe AD who are taking CIBINQO, with or without Rx topical therapies.

The Long Term Extension trial (JADE EXTEND) is an ongoing safety study for eligible subjects taking CIBINQO who have completed a qualifying JADE parent study.
* Subjects could continue to receive CIBINQO in the LTE trial until availability of commercial CIBINQO or until the study was terminated in their respective country.
- Patients assessed in JADE EXTEND were allowed to use topical medications as needed
Primary (safety) endpoint
- Long-term safety:
- study duration
- incidence of serious AEs and AEs leading to discontinuation
- incidence of clinical abnormalities and change from baseline in clinical laboratory values, ECG measurements, and vital signs
Biases
- LTE studies may provide useful data on low-frequency, long-latency AEs, risk factor analysis, and trends over exposure time. However, conduct of LTE studies in which both treatment and dose are known to both investigator and patient is subject to certain biases and limitations, and therefore data should be interpreted with caution
- Biases include, but are not limited to:
- patient selection (patient willingness or ineligibility to enroll, which may be due to prior serious AEs)
- prior treatment and investigator/patient expectation
- volunteer, observer, and responder/survivor effects
- initial dose of study drug
- incidence of treatment-emergent AEs
Limitations
- Limitations include, but are not limited to:
- AE frequencies and incidence rates subject to change over time due to patient entry/exit
- dose changes or study drug interruptions influenced by both investigator and patient
- the number of patients and exposure for a specific safety event possibly differing depending on the timing of censored events
- the number of observed patients with longer exposure times becoming lower
AD=atopic dermatitis.
JADE EXTEND inclusion/ exclusion criteria1
Inclusion criteria
- ≥12 years of age*
- Body weight ≥88 lbs if applicable from qualifying JADE parent study
- Completed the full treatment or full rescue treatment of a qualifying parent study, or completed the full open-label run-in on REGIMEN but did not meet response criteria
Exclusion criteria
- Active forms of other inflammatory skin diseases
- Presence of other medical conditions at the discretion of the investigator
- Pregnant or breastfeeding women
- Women of childbearing potential who are unwilling to use contraception
- Discontinued from treatment (or rescue treatment) early in a qualifying parent study
- Ongoing adverse event in qualifying parent study
* Adolescent subjects below the age of 18 years old (or country-specific age of majority) will only be enrolled in this study if instructed by the sponsor and approved by the country or regulatory/health authority. If these approvals have not been granted, only subjects aged 18 years (or country-specific age of majority) and older will be enrolled.
SERIOUS INFECTIONS
Serious infections have been reported in patients receiving abrocitinib. The most frequent serious infections in clinical studies were herpes simplex, herpes zoster and pneumonia.
Treatment must not be initiated in patients with an active, serious systemic infection.
Risks and benefits of treatment prior to initiating abrocitinib should be considered for patients:
- with chronic or recurrent infection
- who have been exposed to TB
- with a history of a serious or an opportunistic infection
- who have resided or travelled in areas of endemic TB or endemic mycoses; or
- with underlying conditions that may predispose them to infection.
MALIGNANCY
Malignancies, including non-melanoma skin cancer (NMSC), were observed in clinical studies with abrocitinib. Clinical data are insufficient to assess the potential relationship of exposure to abrocitinib and the development of malignancies. Long-term safety evaluations are ongoing.
The risks and benefits of abrocitinib treatment should be considered prior to initiating in patients with a known malignancy other than a successfully treated NMSC or cervical cancer in situ or when considering continuing therapy in patients who develop a malignancy. Periodic skin examination is recommended for patients who are at increased risk for skin cancer.
LIPIDS
Dose-dependent increases in blood lipid parameters were reported in patients treated with abrocitinib compared to placebo. Lipid parameters should be assessed approximately 4 weeks following initiation of therapy and thereafter according to the patient’s risk for cardiovascular disease. The effect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined. Patients with abnormal lipid parameters should be further monitored and managed according to clinical guidelines, due to the known cardiovascular risks associated with hyperlipidaemia. In patients with a high burden of cardiovascular risk factors, the risks and benefits of abrocitinib compared to that of other available therapies for atopic dermatitis should be considered. If abrocitinib is chosen, interventions to manage lipid concentrations should be implemented according to clinical guidelines.
Thrombotic events including pulmonary embolism
Events of deep venous thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients receiving abrocitinib. Abrocitinib should be used with caution in patients at high risk for DVT/PE. Risk factors that should be considered in determining the patient's risk for DVT/PE include older age, obesity, a medical history of DVT/PE, prothrombotic disorder, use of combined hormonal contraceptives or hormone replacement therapy, patients undergoing major surgery or prolonged immobilisation. If clinical features of DVT/PE occur, treatment should be discontinued and patients should be evaluated promptly, followed by appropriate treatment.
Other warnings include potential laboratory abnormalities. Avoid use of live vaccines during or immediately prior to treatment.

Malignancy
- Excluding NMSC: No cases were reported in subjects treated with placebo or either dose of CIBINQO
- NMSC: 1 subject treated with CIBINQO 200 mg
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The size of the control groups and duration of treatment do not permit precise comparative assessments for AEs with low frequency or long latency. In the chart where certain AEs have a zero, indicating no cases were observed in the reporting period, this should not be interpreted as no risk for a specified event.
Data shown includes one phase 2b and three phase 3 trials.
* Exposure adjusted incidence rates (IRs) were adjusted by trial for all the adverse reactions reported in this section. Patient-year is defined as the aggregate total follow-up time calculated for each event, censored at the time of first event for any given patient, subject to a risk period of up to 28 days beyond the last dose or to the data cutoff date.
† Opportunistic infections were generally cases of multidermatomal cutaneous herpes zoster.
‡ Includes all malignancy, including non-melanoma skin cancer (NMSC). Patients with history of malignancy except for treated NMSC and cervical carcinoma in situ were excluded from enrolling in trials.
§ MACE (major adverse cardiovascular event) defined as cardiovascular death, myocardial infarction, and stroke.
IR=incidence rate; PY=patient-years.
Well-studied safety profile in 3582 patients across multiple clinical trials, with some having exposure for ~2 years1-3
A long-term extension (LTE) safety study evaluating adults and adolescents aged 12 and older with moderate-to-severe AD who are taking CIBINQO, with or without Rx topical therapies.2
AEs of special interest
These data are from an integrated safety analysis consistent-dose cohort (n=2784) of JADE EXTEND; there was also a variable dose cohort that included additional safety data (n=798). 1451 patients treated with CIBINQO had at least 48 weeks of exposure, and 554 patients had at least 96 weeks of exposure. Data cutoff was April 16, 2021.*

Patients receiving placebo were eligible to enroll in JADE EXTEND following completion of the qualifying JADE parent study; therefore, no patient received placebo after week 16.
Data shown includes one phase 2b and three phase 3 trials.
* Pooled safety from one phase 2b trial, six phase 3 trials, and one long-term extension (LTE) trial, which is ongoing. In the consistent-dose cohort, patients received the same CIBINQO dose during the entire exposure time. In the variable-dose cohort, patients received different doses of CIBINQO (100 mg or 200 mg) throughout the exposure time.
† Exposure-adjusted IRs were adjusted by trial for all of the adverse reactions reported in this section. Patient-year is defined as the aggregate total follow-up time calculated for each event, censored at the time of first event for any given patient, subject to a risk period of up to 28 days beyond the last dose or to the data cutoff date.
‡ Opportunistic infections were generally cases of nonserious multidermatomal herpes zoster. There was 1 event of adjudicated tuberculosis.
§ Patients with a history of malignancy, except for treated NMSC and cervical carcinoma in situ, were excluded from enrolling in the trials.
‖ MACE was defined as cardiovascular death, myocardial infarction, and stroke.
¶ One pulmonary embolism event (not adjudicated) was included.
# Confidence intervals (Cis) reflect the uncertainty around an estimated number; therefore, the upper limit (UL) is nonzero, reflecting that there is a chance that the true underlying IR could be slightly greater than zero with only a 2.5% chance (due to 95% CI) to be >0.47 for the incidence of PE and DVT in the CIBINQO 100 mg group, or >0.57 for the incidence of retinal detachment in the CIBINQO 200 mg group.
AE=adverse event; DVT=deep vein thrombosis; IR=incidence rate; MACE=major adverse cardiovascular event; NMSC=non-melanoma skin cancer; PY=patient-years.
JADE EXTEND study design1,2
A long-term extension (LTE) safety study evaluating adults and adolescents aged 12 and older with moderate-to-severe AD who are taking CIBINQO, with or without Rx topical therapies.

The Long Term Extension trial (JADE EXTEND) is an ongoing safety study for eligible subjects taking CIBINQO who have completed a qualifying JADE parent study.
* Subjects could continue to receive CIBINQO in the LTE trial until availability of commercial CIBINQO or until the study was terminated in their respective country.
- Patients assessed in JADE EXTEND were allowed to use topical medications as needed
Primary (safety) endpoint
- Long-term safety:
- study duration
- incidence of serious AEs and AEs leading to discontinuation
- incidence of clinical abnormalities and change from baseline in clinical laboratory values, ECG measurements, and vital signs
Biases
- LTE studies may provide useful data on low-frequency, long-latency AEs, risk factor analysis, and trends over exposure time. However, conduct of LTE studies in which both treatment and dose are known to both investigator and patient is subject to certain biases and limitations, and therefore data should be interpreted with caution
- Biases include, but are not limited to:
- patient selection (patient willingness or ineligibility to enroll, which may be due to prior serious AEs)
- prior treatment and investigator/patient expectation
- volunteer, observer, and responder/survivor effects
- initial dose of study drug
- incidence of treatment-emergent AEs
Limitations
- Limitations include, but are not limited to:
- AE frequencies and incidence rates subject to change over time due to patient entry/exit
- dose changes or study drug interruptions influenced by both investigator and patient
- the number of patients and exposure for a specific safety event possibly differing depending on the timing of censored events
- the number of observed patients with longer exposure times becoming lower
AD=atopic dermatitis.
JADE EXTEND inclusion/ exclusion criteria1
Inclusion criteria
- ≥12 years of age*
- Body weight ≥88 lbs if applicable from qualifying JADE parent study
- Completed the full treatment or full rescue treatment of a qualifying parent study, or completed the full open-label run-in on REGIMEN but did not meet response criteria
Exclusion criteria
- Active forms of other inflammatory skin diseases
- Presence of other medical conditions at the discretion of the investigator
- Pregnant or breastfeeding women
- Women of childbearing potential who are unwilling to use contraception
- Discontinued from treatment (or rescue treatment) early in a qualifying parent study
- Ongoing adverse event in qualifying parent study
* Adolescent subjects below the age of 18 years old (or country-specific age of majority) will only be enrolled in this study if instructed by the sponsor and approved by the country or regulatory/health authority. If these approvals have not been granted, only subjects aged 18 years (or country-specific age of majority) and older will be enrolled.
References:
- Cibinqo (abrocitinib) latest approved Isareli prescribing information.
- Data on file. Pfizer Inc; New York, NY.
- Simpson EL, Silverberg JI, Nosbaum A, et al. Integrated safety analysis of abrocitinib for the treatment of moderate-to-severe atopic dermatitis from the phase II and phase III clinical trial program. Am J Clin Dermatol. 2021;22(5):693-707.

